Why Investors Need to Do More Rigorous Medical Diligence as a Core Part of any Healthtech Investment
Yesterday’s big news, reported by Chrissy Farr of CNBC, was ‘“Gut health start-up uBiome files for bankruptcy five months after FBI raid.” In other words, yet another health tech company is making headlines for avoidable, costly—or dangerous—missteps. The troubles of uBiome, Theranos, Nurx and 23andMe have been high profile enough to make the press, but they are by no means unusual. In fact, similar missteps are happening all the time with smaller health tech companies, usually unbeknown to their investors.
It’s time that we in the industry, who are creating the health products of the future, ask ourselves why these problems keep happening and what we need to do to fix them. A heated debate erupted on Twitter in response to the latest uBiome headlines, questioning what role investors play in enabling missteps and whether they are doing an appropriate level of medical diligence when they fund health tech companies.
Investing in early-stage companies is always risky particularly in the unproven health tech sector. In every investment transaction, investors conduct a process of due diligence to confirm the accuracy of claims about the company’s finances, business model and teams. The due diligence process is intended to help identify the potential winners, elucidate key risks, and develop a risk mitigation plan with the management team. Common areas of review during the diligence process include finances, operations, legal, and, when appropriate, technical. Although the investors and entrepreneurs need to be aware of diligence issues, the actual assessment is typically done by professionals—accountants for financial diligence, lawyers for legal diligence, and so forth.This is because these individuals do a far better job of identifying potential pitfalls or risks.
Yet in health tech, the process happens in a different way. When it comes to the medical diligence required to evaluate a company, tech investors usually rely on their associates instead of on professionals with medical industry expertise.
Medical Diligence Is Missing
We would hope that by simply presenting the above sentence to the investor world, the logical disconnect would speak for itself. In case that’s not convincing enough, the proof of the problem is in the numbers: Over $50 billion has been invested in health tech in the last ten years, but with very few successful exits. Clearly, most medical diligence that has been done to date has not been rigorous enough. For the most part, it has failed to separate those companies who are creating a clinically and commercially viable health product from those who are not. And it has failed to detect the ones making the egregious missteps we are seeing in the headlines.
Tech entrepreneurs are famous for solving problems with rapid iteration and learning. However, they can often take far too long—five years or more—to discover certain requirements for success in health tech: Specifically, that:
-
There is a formula for achieving widespread market access in health care
-
They need team members who are part of the healthcare industry
-
Credible evidence presented in the right places is necessary before widespread adoption of a new health product can happen.
Proper medical diligence that takes these principles into account can save entrepreneurs years in opportunity cost and save investors tens of millions of dollars per company.
Investors Need to Lead
The investment community has the power to propel the health tech industry forward and accelerate the disruption of health care by identifying the most viable health products faster. In order to do this effectively, investors need to know that a company’s business practices and financing are sound. But they also need to know if a product or service is actually clinically useful, solves a real healthcare challenge, and whether the company has data or is conducting studies that support its claims and stated value propositions. The timeframe to investors’ return on investment is overtly tied to the answers to these questions. Rigorous medical diligence is ESSENTIAL to investor success and to the creative disruption of healthcare.
While the elements of medical diligence are well known to traditional healthcare investors, we believe it’s important for all investors to obtain an assessment of these criteria when considering an investment in a health tech company. We have outlined these essential parameters in our blog The Formula for Widespread Adoption of Health Products that Every Investor and Health Tech Entrepreneur Needs to Know.
At MDisrupt, we believe that an obvious solution to the problems in health tech is to engage healthcare professionals and market access experts to assess the viability of health tech investments. As with legal, financial, and technical diligence, medical diligence, when conducted by experienced professionals, can save time and money, avoid embarrassing missteps, and set appropriate revenue timeline expectations.
MDisrupt works with investors and health tech entrepreneurs to do an independent, transparent, and objective assessment of the clinical and commercial viability of health-related products and services. We can identify red flags early on as well as work with companies to bridge any gaps they may have. Our assessment includes:
-
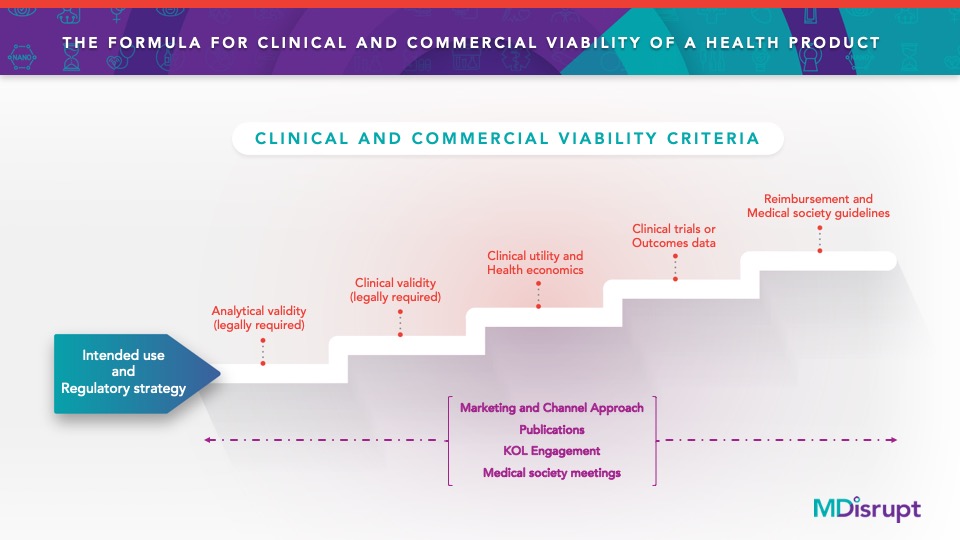
Clinical Viability
-
Intended use and product-market fit
-
Analytical and clinical validity
-
Clinical utility and health economic models
-
Prospective outcomes studies
-
-
Commercial Viability
-
Coding, coverage, and reimbursement, if appropriate
-
Clinical dossier development
-
Key opinion leader strategy and eventual inclusion in professional society guidelines
-
Channel optimization and market access strategy
-
-
Regulatory Strategy
-
Privacy and Security
As an industry, we need to do better. We need to combine the best business philosophies of the tech industry with the best practices of the healthcare industry to help get the most impactful products to patients faster. This is MDisrupt’s mission.
If you are an investor considering an investment in a health tech company, talk to us. We are happy to outline and explain the essential elements of medical diligence and why they are important to successful investments. Medical diligence can help keep you and your portfolio companies from making headlines for the wrong reasons.

Jill Hagenkord, MD
MDisrupt Guest Author
Jill is a board-certified pathologist with subspecialty boards in molecular genetic pathology and a fellowship in pathology/oncology informatics. She brings expertise in health product strategy, coding, coverage, reimbursement, medical and regulatory affairs, health policy, clinical laboratory medicine, population health, provider education and patient engagement.
Every health tech company wants widespread adoption for its health product. There is a community of healthcare experts who would love to help you. Talk to us—we can help.