Building a health product or service that will gain widespread adoption requires a long term plan and thoughtful orchestration between your Medical Affairs and Commercial teams. While we show you the formula below, each step of strategy and execution are intertwined over time as milestones are achieved.
This formula is well-known and well-established for traditional healthcare investors and entrepreneurs, but may be less familiar to those from the tech industry. Health tech entrepreneurs are often tempted to skip a step or delude themselves into believing that their product will be so impactful that the medical industrial complex will not require evidence of safety and utility. They are wrong. None of the steps we outline below can be skipped. Instead, entrepreneurial creativity and technology can and should be harnessed to achieve these milestones more quickly than ever before. Combining innovative experts from health and tech increases your chances of getting widespread adoption more quickly.
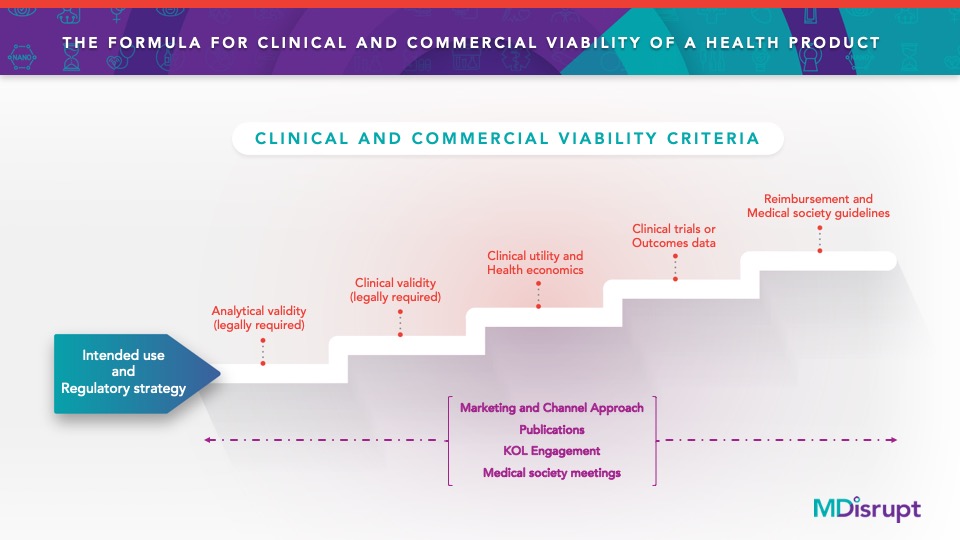
Above: The necessary steps for widespread adoption of a health product.
Define Your Product’s Intended Use.
Your company will need a statement that clearly describes what the device/test/software is testing, the technology it relies on, why the testing is performed, acceptable sample types (if relevant), and who is or is not an appropriate test subject. This statement guides the performance specifications and development process. It also determines the risk level and complexity of the product, which impacts the regulatory requirements. It is the “North Star” that provides clarity to the product, marketing, medical/scientific affairs, legal, regulatory, and engineering teams.
Meet Regulatory Requirements for Market Entry (this is about the device/test/software itself)
- Analytical Validity: The device/test/software must accurately detect the analyte when this substance is present (analytical sensitivity) and not indicate that it is present when it is not (analytical specificity).
- Clinical Validity: The test must accurately detect the disease or condition when it is known to be present (clinical sensitivity) and not indicate that it is present it when it is not (clinical specificity).
Demonstrate Proof Points Necessary for Widespread Adoption (this is about the intervention)
Interventions can be medicinal, surgical, or behavioral. Whatever category the intervention falls into, it must be valid, beneficial, and impactful relative to cost and alternatives.
- Clinical Utility: Based on the results of the device/test/software, there is an intervention that has been shown to be safe and effective in the test population.
- Health Economic Model: There is a budget impact, cost effectiveness, or similar model to estimate the relative health and economic benefits of the intervention.
- Prospective Outcomes Research: How safe and effective is the test and intervention in real people in real time? This can be established with a randomized clinical trial or real-world evidence collection. It is possible to negotiate Risk Sharing Agreements (eg, Coverage with Evidence Development) with forward-thinking payers to obtain conditional payment for this study.
- Professional Society Guidelines: It is important to have a credible, external, academic medical champion(s) for your health product. Involve this person deeply in all steps described above so that they understand the product and the data to support its adoption into medical professional society guidelines. For liability reasons, most physicians adhere to society guidelines most of the time, so these guidelines are key to acceptance and adoption. A champion who is a research scientist, while he or she may be credible and highly accomplished, is not sufficient; medical training and scientific training are drastically different and provide very different perspectives on the viability of a health product.
- Select the Right Market Access and Commercial Strategy: If it is desirable to get a test covered by insurance, that requires a somewhat different path than self pay or institutional pay. However, all paths will require specific evidence presented in the right way to the right channel at the right time. Even for a self-pay strategy, consumers will bring the results of a health product to their healthcare provider. Failing to earn the trust of the healthcare community is a common oversight for health tech companies. It is important to close the loop on the customer experience as they interact with their care providers.
The medical community is THE most skeptical audience a marketer can encounter. Those in this sector are trained to make data-based decisions. Knowing this, there is an art and a science to timely communication with this audience and showcasing data-driven messages using the appropriate media, channels and spokespeople. Healthcare marketers know how to effectively communicate with medical audiences through a sophisticated combination of engaging their internal medical affairs colleagues, KOLs (Key opinion leaders), and medical societies. They are also experts at generating appropriate marketing materials that can sway even the most cynical medical audiences,
Every health tech company wants widespread adoption for its health product and, as you can see, there is a path to getting there. There is also a community of healthcare experts who would love to help. MDisrupt is the conduit.
Jill Hagenkord, MD
MDisrupt Guest Author
Jill is a board-certified pathologist with subspecialty boards in molecular genetic pathology and a fellowship in pathology/oncology informatics. She brings expertise in health product strategy, coding, coverage, reimbursement, medical and regulatory affairs, health policy, clinical laboratory medicine, population health, provider education and patient engagement.
Every health tech company wants widespread adoption for its health product. There is a community of healthcare experts who would love to help you. Talk to us—we can help.
